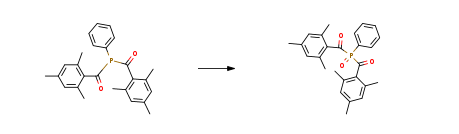OMNIRAD 819 CAS#: 162881-26-7; ChemWhat Code: 69404
Identification
| Product Name | OMNIRAD 819 |
| IUPAC Name | [phenyl-(2,4,6-trimethylbenzoyl)phosphoryl]-(2,4,6-trimethylphenyl)methanone |
| Molecular Structure |  |
| CAS Registry Number | 162881-26-7 |
| EINECS Number | 423-340-5 |
| MDL Number | MFCD01863675 |
| Synonyms | phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide, phenyl bis(2,4,6?trimethylbenzoyl)phosphine oxide, bis (2,4,6-trimethylbenzoyl) phenylphosphine oxide, bis(2,4,6-trimethylbenzoyl)-phenyl-phosphine oxide, phenyl-bis(2,4,6-trimethylbenzoyl)phosphine oxide, bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide, phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide; CAS NO.: 162881-26-7; CAS Number: 162881-26-7 |
| Molecular Formula | [(CH3)3C6H2CO]2P(O)C6H5 |
| Molecular Weight | 418.46 |
| InChI | InChI=1S/C26H27O3P/c1-16-12-18(3)23(19(4)13-16)25(27)30(29,22-10-8-7-9-11-22)26(28)24-20(5)14-17(2)15-21(24)6/h7-15H,1-6H3 |
| InChI Key | GUCYFKSBFREPBC-UHFFFAOYSA-N |
| Canonical SMILES | CC1=CC(=C(C(=C1)C)C(=O)P(=O)(C2=CC=CC=C2)C(=O)C3=C(C=C(C=C3C)C)C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110452259 | Method for initiating hydrosilylation reaction (by machine translation) | 2019 |
| CN103288873 | Sulfonyl or quinonyl functionalized acyl phosphine oxidation compound (by machine translation) | 2017 |
| CN105884809 | Acylphosphanes (oxygen) or sulfonamido diphenylphosphinobiphenyl (oxygen) preparation method of compound (by machine translation) | 2016 |
| US8507584 | Phase change inks containing amide gellant compounds with aromatic end groups | 2013 |
| US5942290 | Molecular complex compounds of acylphosphine oxide and α-hydroxy ketones as photoinitiators | 1999 |
Physical Data
| Appearance | Yellowish Powder |
| Solubility | acetone, acetonitrile, toluene, and hexanedioldiacrylate: soluble |
| Boiling Point | 590.0±60.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| Melting Point, °C |
| 117 |
| 121 |
| 130 – 131 |
| 127 – 133 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 13C | chloroform-d1 | 100 |
| Chemical shifts | 31P | chloroform-d1 | 162 |
| Chemical shifts, Spectrum | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | CDCl3 | |
| Chemical shifts | 13C | CDCl3 | |
| Chemical shifts | 31P | CDCl3 |
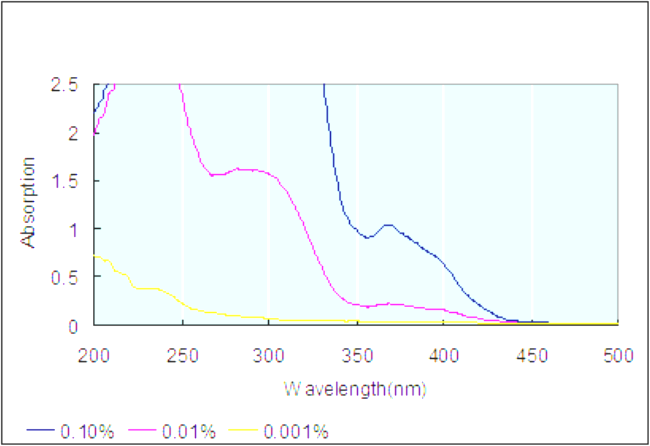
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Spectrum | dichloromethane | 405 | 500 |
| Spectrum | chloroform | ||
| 370, 405, 455, 470 | 950, 500 | ||
| Spectrum | benzene | ||
| Band assignment, Spectrum | 307 | ||
| methanol | 366 | 1059 | |
| Spectrum | dimethyl sulfoxide | ||
| N,N-dimethyl-formamide | 290, 365, 395 | ||
| Spectrum | ethylbenzene | 371, 396 | 727, 919 |
| acetonitrile | 369 | 8820 |
Route of Synthesis (ROS)
| Conditions | Yield |
| With Nitrogen dioxide In toluene for 1.5h; Inert atmosphere; Experimental Procedure Example 2 (3) the reaction flask cooled to 50 ° C, the reaction flask to pass excess nitrogen dioxide gas, stirring the reaction solution to keep the reaction temperature constant, the intermediate product bis (2,4,6 _ – trimethyl (2, 4, 6-trimethylbenzoyl) phenyl phosphine oxide, while generating nitric oxide; passing nitric oxide gas into a gas recovery column, and oxidizing the nitric oxide gas, The tower was filled with enough air to re-oxidize nitric oxide to produce nitrogen dioxide and continue into the reaction bottle for circulation until the reaction is complete; continue stirring for 1.5 hours to complete the reaction and gas spill, stop the reaction, The excess nitrogen dioxide gas with sodium hydroxide solution absorption; (4) The reaction was stopped, separated and extracted, and the organic phase was washed twice with 250 ml of pure water, dried over anhydrous sodium sulfate, The toluene solvent was distilled off under reduced pressure to obtain a yellow target 5) The crude product was recrystallized from hexane to give a light yellow solid, which was 79.2g of the pure product of the target photoinitiator 819. The photoinitiator 819 synthesized by this method was analyzed by PLC for purity over 99.0% Phenyl phosphine dichloride, the yield was 94.7% | 94.7% |
| With dihydrogen peroxide In toluene at 45 – 50℃; | |
| With dihydrogen peroxide In water; chlorobenzene; toluene; tert-butyl alcohol at 40 – 50℃; for 2h; Solvent; | 53.4g |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H413: May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P272, P273, P280, P302+P352, P321, P333+P313, P363, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | NONH for all modes of transport |
| Under the room temperature and away from light | |
| HS Code | 293190 |
| Storage | It is sensitive to visible light and any exposure to sunlight should be avoided. Kept at low/ambient temperature and dry conditions. Avoid contacting with strong oxidants. |
| Shelf Life | 2 years |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 418.472 |
| logP | 7.601 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 61.02 |
| Rotatable Bond (RotB) | 5 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Dose |
| 4.39 | percentage of necrotic cells | 71.2 | % | 100 μM |
| 3.64 | percentage of necrotic cells | 30.3 | % | 100 μM |
| 1 | percentage of necrotic cells | 19.9 | % | 50 μM |
| 1 | percentage of necrotic cells | 3.7 | % | 5 μM |
| 1 | percentage of early apoptotic cells | 13.3 | % | 25 μM |
| 1 | percentage of early apoptotic cells | 4.9 | % | 100 μM |
| 1 | percentage of early apoptotic cells | 5.4 | % | 50 μM |
| 1 | percentage of early apoptotic cells | 6.6 | % | 5 μM |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 5.3 | inhibition rate | Active | 5~100 μM | ||
| 5.3 | inhibition rate | Active | 5~100 μM | ||
| inhibition rate | – | 100 | % | 50 μM | |
| inhibition rate | – | 100 | % | 50 μM |
| Use Pattern |
| OMNIRAD 819 CAS#: 162881-26-7 Polymers/polymer applications |
| radiation polymerization curing materials |
| Cosmetics/dental/toilet |
| initiator in dental restorative composition |
| free radical photoinitiator |
| photoinitiator |
| Chemical processes/laboratory use |
| Dental adhesive composition |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| IGM Resins | https://www.igmresins.com/ |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |