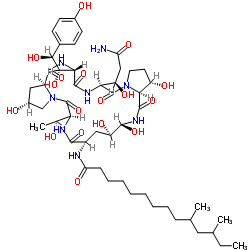
Pneumocandin B0 CAS#: 135575-42-7; ChemWhat Code: 434221
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/58082 | INTERMEDIATE FOR SYNTHESIZING CASPOFUNGIN AND PREPARATION METHOD THEREOF | 2014 |
Physical Data
| Appearance | Off white powder |
| Solubility | No data available |
| Boiling point | 1442.9±65.0 °C(Predicted) |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
| Description (Association (MCS)) | Solvent (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with … | CCl4 | 24.9 | 4-Fluorophenol |
| Stability constant of the complex with … | aq. HNO3 | 25 | AgNO3 |
| Enthalpy of association | acetonitrile | 25 | iodine |
| NMR spectrum of the complex | CDCl3 | Cu(2,4-dichloro-benzoate)2 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| Chemical shifts | 1H | tetradeuteriomethanol | |
| Chemical shifts | 13C | tetradeuteriomethanol | |
| Spin-spin coupling constants | tetradeuteriomethanol | 1H-1H. |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm | Ext./Abs. Coefficient, l·mol-1cm-1 |
| Absorption maxima | methanol | 276 | 15 |
Route of Synthesis (ROS)
| Conditions | Yield |
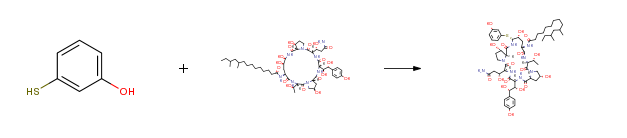
| With trifluoroacetic acid; phenylboronic acid In acetonitrile at -50 – -45℃; for 2.5h; Inert atmosphere; Experimental Procedure Under nitrogen gas protection, acetonitrile (100 ml), the compound of formula (2) (5.0 g), phenylboronic acid (0.90 g) and 3-mercaptophenol (1.80 g) were uniformly stirred and the temperature was raised to -50 to -45°C And trifluoroacetic acid (1.05 ml) was added dropwise. After the dropwise addition, the mixture was reacted at -50 to -45°C for about 2.5 hours. After confirming the completion of the reaction by TLC monitoring, the reaction was stopped and an aqueous solution of NaOAc (1.15 g NaOAc dissolved in 25 ml of water) was added slowly, and the temperature was raised to 20 ° C and stirred for 2 hours. A large amount of solid was precipitated, the temperature was lowered to 0 ° C or lower, and the filtrate was subjected to third washing with 60 ml of acetonitrile / water = 9: 1 (V / V), followed by vacuum drying for 5 hours, 3b (4.65 g, yield 93%). | 93% |
| With trifluorormethanesulfonic acid; phenylboronic acid In acetonitrile at -50 – -45℃; for 2.5h; Inert atmosphere; | 0.27 |
Safety and Hazards
| Pictogram(s) |     |
| Signal | Danger |
| GHS Hazard Statements | H315 (92.31%): Causes skin irritation [Warning Skin corrosion/irritation] H317 (15.38%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (92.31%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H334 (15.38%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] H335 (15.38%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] H400 (92.31%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P271, P272, P273, P280, P285, P302+P352, P304+P340, P304+P341, P305+P351+P338, P310, P312, P321, P332+P313, P333+P313, P342+P311, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerous goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 1065.23 |
| logP | 3.701 |
| HBA | 24 |
| HBD | 15 |
| Matching Lipinski Rules | 1 |
| Veber rules component | |
| Polar Surface Area (PSA) | 411.28 |
| Rotatable Bond (RotB) | 21 |
| Matching Veber Rules | 0 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Biological Species | Target | Bioassay | Effect |
| 7.25 | MFC (minimum fungicidal concentration)(Antifungal activity) | = | 0.06 | µg/mL | Candida tropicalis | |||
| 7.15 | IC50 | 70 | nM | In Vitro (others) | antifungal agent | |||
| 7.15 | IC50 | = | 0.07 | µM | Candida albicans | Inhibitor | ||
| 7.15 | IC50(Beta-(1,3)-D-glucan synthesis) | = | 70 | nM | Candida albicans MY1208 | Inhibitor | ||
| 6.95 | MFC (minimum fungicidal concentration)(Minimum Inhibitory Concentration) | = | 0.12 | µg/mL | Candida tropicalis MY1012 | |||
| 6.63 | MFC (minimum fungicidal concentration) | = | 0.25 | μg/ml | Candida albicans | |||
| 6.63 | MFC (minimum fungicidal concentration) | = | 0.25 | μg/ml | Candida albicans MY1208 | |||
| 6.63 | MFC (minimum fungicidal concentration)(Minimum Inhibitory Concentration) | = | 0.5 | μg/ml | Candida albicans MY1055 | |||
| 6.03 | MFC (minimum fungicidal concentration) | = | 1 | μg/ml | Kluyveromyces marxianus MY2099 | |||
| 5.73 | MFC (minimum fungicidal concentration)(Minimum Inhibitory Concentration) | = | 2 | µg/mL | Candida parapsilosis MY1010 | |||
| 5.42 | MFC (minimum fungicidal concentration)(Antifungal activity) | = | 4 | µg/mL | Candida parapsilosis | |||
| 4.69 | IC50 | 20.23 | µM | In Vitro (others) | ||||
| 3.92 | MIC | 128 | mg/L | In Vitro (others) | fungistatic agent | |||
| ZI (zone of inhibition) | 8 | mm | In Vitro (others) | fungistatic agent | ||||
| MED99.9(Reduction (99.9%) of viable CFUs recoverable from mouse kidneys infected with Candida albicans (MY1208)) | = | 6 | mg/kg | mouse |
| Quantitative Results | ||
| 1 of 9 | Effect | fungistatic agent |
| Biological material | Aspergillus fumigatus | |
| Assay Description | Bioassay : 35 deg C; potato dextrose agar 16 clinical strains (MF5668, CLY315, CLY522, CLY523 among them); in vitro; agar dilution diffusion method (Acar, J.F. 1986. Disk susceptibility test, p. 27-63. In V. Lorian (ed.) Antibiotics in laboratory medicine); 1.2E5 CFU/ml inoculum | |
| Results | critical concentration, CC 0.03 – 0.05 mg/l | |
| 2 of 9 | Effect | Hemolytic |
| Assay Description | Target : CD-1 mouse whole blood Bioassay : unwashed erythrocyte hemolysis assay; room temperature | |
| Results | MLC, minimum lytic concentration active 3.13 mg/l | |
| 3 of 9 | Effect | Hemolytic |
| Biological material | human | |
| Assay Description | Bioassay : unwashed erythrocyte hemolysis assay; room temperature | |
| Results | MLC, minimum lytic concentration active 25 mg/l | |
| 4 of 9 | Effect | antifungal agent |
| Assay Description | Target : Candida albicans MY1055 Bioassay : time-killing assay | |
| Results | time-kill curve; fungicide | |
| 5 of 9 | Results | glucan synthesis inhibition in a Candida albicans membrane assay: IC50 = 0.07 μM |
| 6 of 9 | Effect | fungistatic agent |
| Assay Description | Target : Aspergillus fumigatus H11-20 Bioassay : in vitro; agar diffusion assay; potato dextrose agar; 1E6 conidia/petri dish inoculum; 30 deg C; zone of inhibition measured | |
| Results | critical conc. < 0.06 mg/l | |
| 7 of 9 | Effect | fungistatic agent |
| Assay Description | Effect : eye observable change in morphology Target : Aspergillus fumigatus H11-20 Bioassay : in vitro; broth microdilution assay; Yeast Nitrogen Base with 2 percent glucose; 1E4 condidia/well inoculum; 30 deg C | |
| Results | min. effective conc., MEC 0.5 mg/l | |
| 8 of 9 | Effect | Antifungal activity against Candida albicans, Candida tropicalis, Candida parapsilosis, Candida pseudotropicalis |
| 9 of 9 | Results | TOKA ED99.9 = 6 mg/kg |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (quant) | Unit | Biological Species | Bioassay | Effect |
| 7.58 | MFC90 | 0.25 | mg/L | Candida albicans | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 7.28 | MFC90 | 0.5 | mg/L | Candida tropicalis | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 6.95 | MFC (minimum fungicidal concentration) | 0.12 – 0.5 | mg/L | Candida albicans | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 6.68 | MFC90 | 2 | mg/L | Candida glabrata | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 6.63 | MFC (minimum fungicidal concentration) | 0.25 – 2 | mg/L | Candida glabrata | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 6.33 | MFC (minimum fungicidal concentration) | 0.5 – 64 | mg/L | Cryptococcus neoformans | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 6.03 | MFC50 | 1 | mg/L | Kluyveromyces marxianus | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 5.73 | MFC50 | 2 | mg/L | Pichia kudriavzevii | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 5.42 | MFC (minimum fungicidal concentration) | 4-128 | mg/L | Meyerozyma guilliermondii | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 5.18 | MFC90 | 64 | mg/L | Meyerozyma guilliermondii | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 5.12 | MFC (minimum fungicidal concentration) | 8 | mg/L | Clavispora lusitaniae | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 4.88 | MIC90 | 128 | mg/L | Aspergillus niger | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| 3.92 | MIC | 128 | mg/L | Aspergillus flavus | Cell/tumor cell: proliferation/viability/growth | antifungal agent |
| Use Pattern |
| Pneumocandin B0 CAS 135575-42-7 is starting material for production of antifungal cyclohexapeptide compounds. |
| Pneumocandin B0 CAS 135575-42-7 is used as the pharmaceutical intermediates of the Caspofungin which is a lyophilized preparation. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | https://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |