(R)-(-)-2-Amino-1-propanol CAS#: 35320-23-1; ChemWhat Code: 86858
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN116003406 | Heteroaromatic ring oxynitride and preparation method and application thereof | 2023 |
| CN115215799 | Urea multi-target tyrosine kinase inhibitor and various medical applications thereof | 2022 |
| US2021/139454 | FORMAMIDE COMPOUND, PREPARATION METHOD THEREFOR AND APPLICATION THEREOF | 2021 |
| US2020/34708 | NOVEL PROBE COMPOUNDS FOR FLUORESCENCE AND/OR CIRCULAR DICHROISM SENSOR FOR AMINE COMPOUNDS INCLUDING AMINO ALCOHOLS, AND SIMULTANEOUS ANALYSIS METHOD OF FLUORESCENCE AND CIRCULAR DICHROISM | 2020 |
| WO2019/101641 | 2-HETARYLPYRIMIDINE-4-CARBOXAMIDES AS ARYL HYDROCARBON RECEPTOR ANATGONISTS | 2019 |
Physical Data
| Appearance | Slightly yellow to clear liquid |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | chloroform-d1 | |
| Chemical shifts | 1H | dimethylsulfoxide-d6 | 400 |
| Chemical shifts | 1H | chloroform-d1 | 400 |
| Chemical shifts | 1H | CDCl3 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Spectrum | neat (no solvent) |
| Bands | CDCl3 |
| Bands | solid Ar |
| Description (UV/VIS Spectroscopy) | Absorption Maxima (UV/VIS), nm |
| in the presence of organic compounds | 567 |
Route of Synthesis (ROS)
| Conditions | Yield |
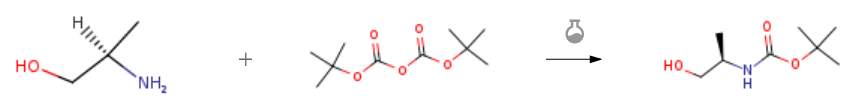
| With triethylamine In methanol at 0 – 20℃; for 12h; Experimental Procedure 4.1.44. tert-Butyl (R)-(1-hydroxypropan-2-yl)carbamate (42) To a solution of (R)-()-2-amino-1- propanol 36 (2.00 g,26.6 mmol) and triethylamine (4.27 mL, 30.6 mmol) in MeOH(20 mL) at 0 C was added di-tert-butyl dicarbonate (6.42 g,29.4 mmol). After stirring at room temperature for 12 h, the solventwas evaporated in vacuo. The residuewas diluted with CH2Cl2. Afterwashing with water, the organic phase was evaporated to drynessfurnishing the title compound (4.60 g, 100%), which was usedwithout further purification in the next step. 1H NMR (300 MHz,CDCl3) d 4.91 (d, J 6.3 Hz, 1H), 3.70 (s, 1H), 3.56 (dt, J 10.8, 5.6 Hz,1H), 3.45 (dd, J 11.5, 5.6 Hz, 2H), 1.39 (s, 9H), 1.10 (d, J 6.8 Hz,3H). 13C NMR (75 MHz, CDCl3) d 156.91 (s), 80.15 (s), 67.46 (s), 49.05(s), 28.99 (s), 17.92 (s). | 100% |
| With sulfonic-acid-functionalized silica In dichloromethane at 20℃; for 0.166667h; | 95% |
| With triethylamine In tetrahydrofuran at 0 – 20℃; for 18h; Experimental Procedure A solution of 10 gm (133 mMol) (R)-2-amino-1-propanol and 14.8 gm (146.5 mMol) triethylamine in 500 mL tetrahydrofuran was cooled to 0° C. To this solution was added all at once 30 gm (133 mMol) di-tert-butyl dicarbonate. The reaction mixture was allowed to stir for about 18 hours at room temperature. The reaction mixture was then concentrated under reduced pressure and the resulting residue was dissolved in 300 mL ethyl acetate. This solution was washed twice with 200 mL of water, once with 200 mL of saturated aqueous sodium chloride, dried over magnesium sulfate and concentrated under reduced pressure to provide 22 gm (94%) of the desired compound as a yellow oil.1H-NMR(CDCl3): δ 4.71 (m, 1H), 3.72 (m, 1H), 3.59-3.46 (m, 2H), 2.86 (m, 1H). | 94% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] |
| Precautionary Statement Codes | P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Store at room temperature, in container tightly sealed; Protect from light. |
| HS Code | |
| Storage | Store at room temperature, in container tightly sealed; Protect from light. |
| Shelf Life | 2 years |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 75.1106 |
| logP | -0.815 |
| HBA | 2 |
| HBD | 2 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 46.25 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Use Pattern |
| (R)-(-)-2-Amino-1-propanol CAS 35320-23-1 as an intermediate in organic synthesis, it is used for the synthesis of emulsifiers, surfactants, dyes, rubber stabilizers, and other organic compounds. In the pharmaceutical field, D-Aminopropanol is commonly used as a pharmaceutical intermediate for the synthesis of various drugs, such as beta-blockers, adrenergic agonists, and others. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Limited | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |