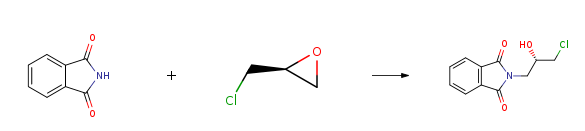
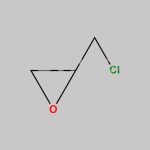
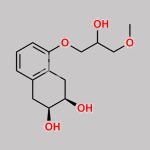
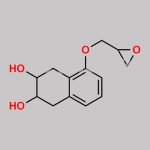
(S)-(+)-Epichlorohydrin CAS#: 67843-74-7; ChemWhat Code: 30667
Identification
| Product Name | (S)-(+)-Epichlorohydrin |
| IUPAC Name | (2S)-2-(chloromethyl)oxirane |
| Molecular Structure | 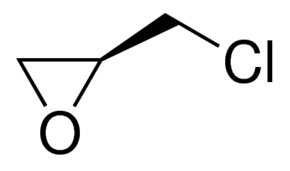 |
| CAS Registry Number | 67843-74-7 |
| EINECS Number | No data available |
| MDL Number | MFCD00077760 |
| Beilstein Registry Number | 1420784 |
| Synonyms | (S)-epichlorohydrin, (S)-(+)-1-chloro-2,3-epoxypropane, (S)-3-chloro-1,2-propylene oxide, (S)-(+)-2-(chloromethyl)oxirane, S-(+)-1-chloro-2,3-epoxypropane, (2S)-3-chloro-1,2-epoxypropane, (S)-(+)-2-chloromethyloxirane;CAS Number: 67843-74-7 |
| Molecular Formula | C3H5ClO |
| Molecular Weight | 92.524 |
| InChI | InChI=1S/C3H5ClO/c4-1-3-2-5-3/h3H,1-2H2/t3-/m1/s1 |
| InChI Key | BRLQWZUYTZBJKN-GSVOUGTGSA-N |
| Canonical SMILES | C1[C@H](O1)CCl |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110885325 | Preparation method (S)- glycidyl-phthalimide (by machine translation) | 2020 |
| CN108440382 | A S – N – glycidol phthalic acid imide preparation method (by machine translation) | 2018 |
| US2013/12532 | CYCLOPROPANECARBOXYLIC ACID DERIVATIVE | 2013 |
| US2014/128601 | PROCESS FOR OBTAINING RIVAROXABAN AND INTERMEDIATE THEREOF | 2014 |
| US2014/206681 | BTK INHIBITORS | 2014 |
Physical Data
| Appearance | Colorless Transparent Liquid |
| Solubility | insoluble |
| Flash Point | 93 °F |
| Refractive index | n20/D 1.438(lit.) |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 25 | 0.25 |
| Density, g·cm-3 | Measurement Temperature, °C |
| 1.0935 | 24.99 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Comment (NMR Spectroscopy) |
| Chemical shifts | 1H | CDCl3 | |
| Spin-spin coupling constants | CDCl3 | 1H-1H |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) |
| Bands | CCl4 |
| Spectrum | neat (no solvent) |
| Spectrum | CS2 |
| Spectrum | CCl4 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) |
| Spectrum | neat (no solvent) |
| Spectrum | CH2Cl2 |
| Spectrum | CHCl3 |
| Spectrum | CS2 |
| Spectrum | CCl4 |
| Spectrum | various solvent(s) |
| Description (Raman Spectroscopy) |
| Spectrum |
Route of Synthesis (ROS)
| Conditions | Yield |
| With N-benzyl-N,N,N-triethylammonium chloride In isopropyl alcohol at 20 – 25℃; for 12h; Reagent/catalyst; | 93.3% |
| With benzyltrimethylammonium chloride In isopropyl alcohol at 15 – 25℃; for 12h; Green chemistry; | 92.7% |
| With potassium carbonate In isopropyl alcohol for 5h; Reflux; | 75% |
| In ethyl acetate | |
| With N-benzyl-N,N,N-triethylammonium chloride In isopropyl alcohol at 43℃; for 12h; Reagent/catalyst; |
Safety and Hazards
| Pictogram(s) |      |
| Signal | Danger |
| GHS Hazard Statements | H226 (98.36%): Flammable liquid and vapor [Warning Flammable liquids] H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H311 (100%): Toxic in contact with skin [Danger Acute toxicity, dermal] H314 (98.36%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H317 (98.36%): May cause an allergic skin reaction [Warning Sensitization, Skin] H330 (14.75%): Fatal if inhaled [Danger Acute toxicity, inhalation] H331 (77.05%): Toxic if inhaled [Danger Acute toxicity, inhalation] H350 (98.36%): May cause cancer [Danger Carcinogenicity] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P272, P280, P281, P284, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P320, P321, P322, P330, P333+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 3(6.1); Packaging Group: II; UN Number: 2023 |
| Under the room temperature and away from light | |
| HS Code | 290389 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price | USD |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 92.5251 |
| logP | 0.612 |
| HBA | 1 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 12.53 |
| Rotatable Bond (RotB) | 1 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Parameter | Value (quant) | Unit |
| Km (Michaelis constant) | 161.4 | mM |
| Vmax | 7.9 | µmol/min/mg |
| kcat | 5.71 | s-1 |
| kcat/Km | 0.035 | mM-1.s-1 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| pX | Parameter | Value (qual) | Value (quant) | Unit | Effect |
| 2.45 | LC50(Lethal dose) | = | 3.58 | mM | Death |
| Use Pattern |
| Used in chiral pharmaceutical intermediates. |
| As a metabolic regulator, used to synthesize fatty acid oxidation inhibitors; |
| For the total synthesis of Macquarimicins and the total synthesis of macrolide RK-397; |
| Chiral structural unit for enantioselective synthesis of hydroxyisoxazolidine and (+)-cis-sylvaticin (a potential antitumor agent) |
| Used for the synthesis of chiral intermediates and chiral drugs |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Caming Pharmaceutical Ltd | http://www.caming.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |