TETRAKIS(DIMETHYLAMINO)ZIRCONIUM CAS#: 19756-04-8; ChemWhat Code: 33708
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| WO2022/169232 | GROUP 4 TRANSITION METAL COMPOUND, METHOD FOR MANUFACTURING SAME, AND METHOD FOR FORMING THIN FILM USING SAME | 2022 |
| KR102080218 | DOUBLE-SUBSTITUTED CYCLOPENTADIENE COMPOUNDS, ORGANOMETALLIC COMPOUNDS AND PREPARATION METHOD THEREOF | 2020 |
| KR102093226 | Silicon-containing organic metal precursor compound, a method for the preparation thereof, and method of manufacturing a silicon metal-oxide thin film | 2020 |
Physical Data
| Appearance | White or yellow crystals |
| Melting Point, °C |
| 57 – 60 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 85 | 0.8 |
| 80 | 0.1 |
| Description (Association (MCS)) |
| SiO2 modified with cyclopentadienylsilylamines |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Chemical shifts | 1H | benzene-d6 | 500 | |
| Chemical shifts | 1H | benzene-d6 | 400 | |
| 13C | further solvent(s) | 25 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | potassium bromide | 27 |
| Spectrum | CCl4 | 14.85 – 54.85 |
Route of Synthesis (ROS)
| Conditions | Yield |
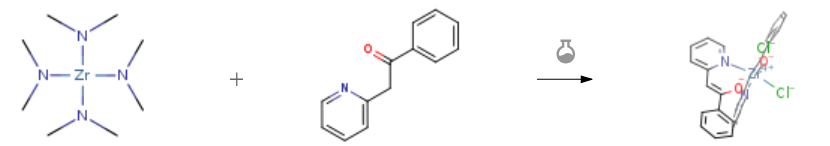
| With chloro-trimethyl-silane In tetrahydrofuran at 20℃; for 5h; Schlenk technique; Inert atmosphere; Experimental Procedure A Schlenk vessel was charged with tetrakis(dimethylamido)zirconium(IV) (135mg, 0.507mmol), dry THF (30mL) and a stirring bar. Under protection from air, 2 (200mg, 1.01mmol) was added. After the solution was stirred at room temperature for 2h, all volatile materials were removed on a vacuum line. To the solid residue, an excess of trimethylchlorosilane (5mL) was added to effect exchange of amido ligands versus chloro ligands. After the mixture was stirred at room temperature for 3h, volatile materials were removed on a vacuum line. The solid residue was triturated with dry n-hexane, filtered off, and dissolved in a small amount of dry dichloromethane. Crystallization afforded the product in the form of yellow needles. Yield: 200mg (71%). M.p. 255-259°C. 1H NMR (300MHz, CD2Cl2, 298K): δ=6.27 (s, 1H), 6.82 (d×d, J=6.30Hz, J=6.44Hz, 1H), 7.13 (d, J=8.03Hz, 1H), 7.50 (m, 3H), 7.59 (d×d, J=7.0Hz, 1H), 7.95 (d, J=5.71Hz, 2H), 8.48 (d, J=5.39Hz, 1H) ppm. 13C NMR (75MHz, CD2Cl2, 298K): δ=104.0, 120.4, 126.2, 126.4, 129.4, 130.6, 136.0, 139.9, 149.2, 155.4, 160.2ppm. IR (ATR): ν=1608, 1541, 1479, 1447, 1364, 1283, 1080, 892, 815, 770, 679, 650cm-1. MS (FAB pos): m/z=532.29 (M-Cl+O)+. Single crystal structure analysis of 15′: Fig. 5 and Table 2. | 71% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H228 (100%): Flammable solid [Danger Flammable solids] H261 (100%): In contact with water releases flammable gas [Danger Substances and mixtures which in contact with water, emit flammable gases] H315 (97.5%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (97.5%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P210, P231+P232, P240, P241, P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P370+P378, P402+P404, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Shelf Life | 1 year |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 267.529 |
| HBA | 4 |
| HBD | 0 |
| Matching Lipinski Rules | 3 |
| Veber rules component | |
| Polar Surface Area (PSA) | 12.96 |
| Rotatable Bond (RotB) | 4 |
| Matching Veber Rules | 2 |
| Use Pattern |
| TETRAKIS(DIMETHYLAMINO)ZIRCONIUM CAS 19756-04-8 may be used as a precursor for synthesizing other zirconium compounds. By controlling reaction conditions, TETRAKIS(DIMETHYLAMINO)ZIRCONIUM is possible to prepare a variety of materials with different properties. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |