Triphenylbismuth CAS#: 603-33-8; ChemWhat Code: 33629
Identification
| Product Name | Triphenylbismuth |
| IUPAC Name | triphenylbismuthane |
| Molecular Structure | 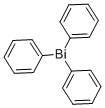 |
| CAS Registry Number | 603-33-8 |
| EINECS Number | 210-033-4 |
| MDL Number | MFCD00014064 |
| Synonyms | TPB, triphenylbismuthane, triphenylbismuthine, tris(phenyl)bismuthane, triphenylbismuth(III), Triphenylbismuthane, triphenyl bismuthin, triphenylbismuthin |
| Molecular Formula | C18H15Bi |
| Molecular Weight | 440.298 |
| InChI | InChI=1S/3C6H5.Bi/c3*1-2-4-6-5-3-1;/h3*1-5H; |
| InChI Key | ZHXAZZQXWJJBHA-UHFFFAOYSA-N |
| Canonical SMILES | C1=CC=C(C=C1)[Bi](C2=CC=CC=C2)C3=CC=CC=C3 |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2015/112104 | Process For Producing Chlorinated Hydrocarbons In The Presence Of A Polyvalent Bismuth Compound | 2015 |
| EP2133357 | LIVING RADICAL POLYMERIZATION PROMOTER | 2009 |
| US2006/148842 | Nepetalactams and N-substituted derivatives thereof | 2006 |
| US4225517 | Process for the production of acetaldehyde by the reaction of methanol with synthesis gas | 1980 |
| US4364872 | Method of making aluminum alkyls | 1982 |
Physical Data
| Appearance | Off-white to white crystal |
| Water Solubility | Insoluble |
| Flash Point | 242°C/14mm |
| Sensitivity | Moisture Sensitive |
| Melting Point, °C | Solvent (Melting Point) |
| 76 | diethyl ether |
| 79 | neat (no solvent) |
| 77-78 | petroleum ether |
| 77 – 78 | methanol, ethanol |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 208 | 0.07 |
| 242 | 14 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.5736 – 1.6427 | 4 | 98.5 – 155 |
| 1.715 | 4 | 75 |
| 1.5851 | 20 |
| Description (Adsorption (MCS)) | Partner (Adsorption (MCS)) |
| Adsorption to title compound | DMF |
| Adsorption to title compound | DMOS |
| Adsorption to title compound | propylene carbonate |
| Adsorption to title compound | acetonitrile |
| Adsorption to title compound | methanol |
| Adsorption to title compound | N-methylformamide |
| Adsorption to title compound | formamide |
| Description (Association (MCS)) | Temperature (Association (MCS)), °C | Partner (Association (MCS)) |
| Stability constant of the complex with | 24.9 | LiC6H5*O(C2H5)2 |
Spectra
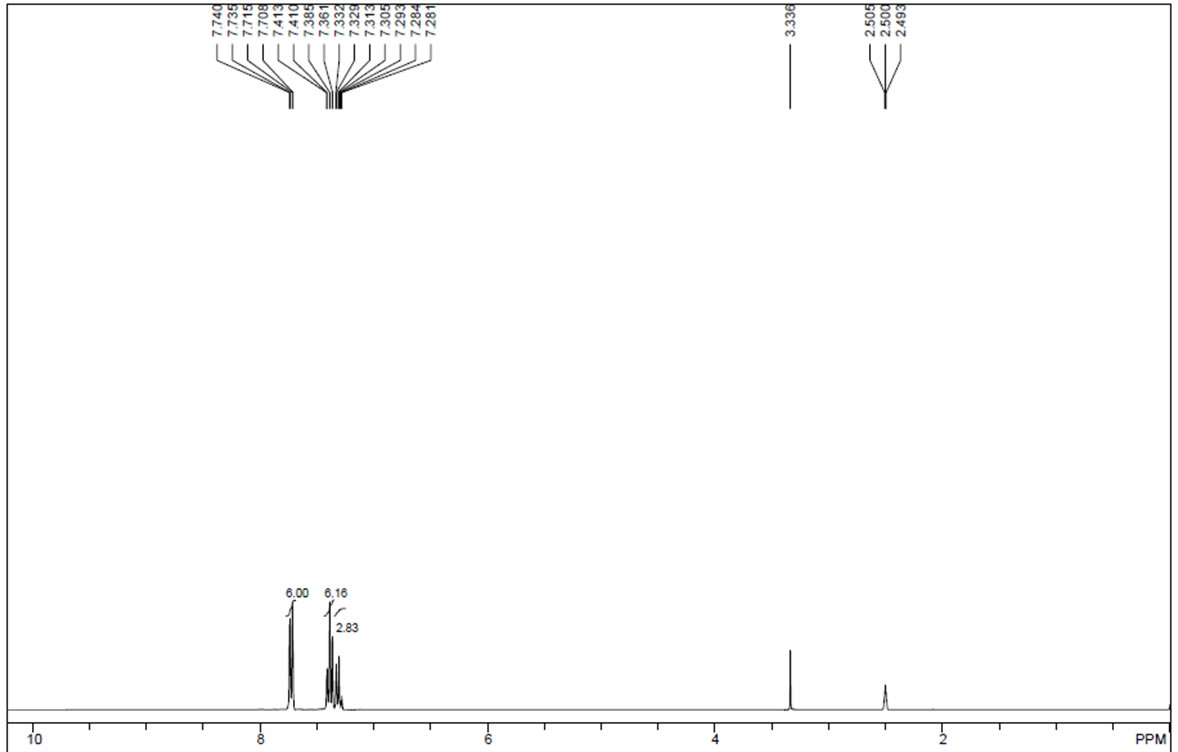
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C | Frequency (NMR Spectroscopy), MHz |
| Spectrum | 1H | chloroform-d1 | 20 | |
| Chemical shifts, Spectrum | 1H | dichloromethane-d2 | 26.84 | 250.1 |
| Chemical shifts, Spectrum | 13C | dichloromethane-d2 | 25.04 | 62.9 |
| Chemical shifts | 13C | chloroform-d1 | 125 |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Bands | gaseous matrix | 30-400 |
| Spectrum | KBr | 30 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Band assignment | not given | 251 nm – 282 nm |
| Spectrum, Band assignment | further solvent(s) | 300 nm – 500 nm |
| Spectrum | ethanol | 200 – 300 nm |
Route of Synthesis (ROS)
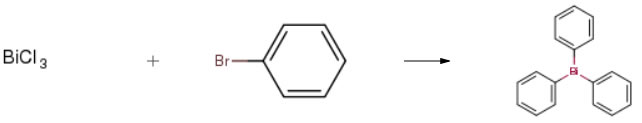
| Conditions | Yield |
| Stage #1: bromobenzene With magnesium In tetrahydrofuran Inert atmosphere; Reflux; Stage #2: bismuth(III) chloride In tetrahydrofuran at -10 – 65℃; for 1.5h; Inert atmosphere; | 83% |
| Stage #1: bromobenzene With magnesium In tetrahydrofuran at 20 – 70℃; Cooling with ice; Inert atmosphere; Schlenk technique; Stage #2: bismuth(III) chloride In tetrahydrofuran at 0 – 20℃; Inert atmosphere; Schlenk technique; Experimental Procedure General procedure: In a typical experiment, 0.5mmol of nitroarene and 0.002g(2mol%) NiNPs/DNA were added to 2mL water and thenstirred for 2-3min for thoroughly mixing. Subsequently,1mmol of NaBH4was added to the reaction mixture undermagnetic stirring at room temperature. The extent of thereaction was monitored by thin layer chromatography.Reproducibility of the results was checked by repeating theruns at least three times and was found to be within acceptablelimits (± 3%). When the reaction was completed, thereaction mixture was diluted with ethyl acetate and the catalystwas recovered by centrifugation. The combined organicfractions were dried over Na2SO4and evaporated underreduced pressure. The crude product was purified by columnchromatography on silica gel with a mixture of ethyl acetateand n-hexane as the eluent, and the ratio of ethyl acetate andn-hexane was depended on the structure of the products.The structure of isolated products was verified by 1H NMR. | 82% |
| Stage #1: bromobenzene With magnesium In acetone; benzene for 1h; Reflux; Inert atmosphere; Stage #2: bismuth(III) chloride In acetone; benzene for 4.5h; Reflux; Inert atmosphere; | 68.2% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Danger |
| GHS Hazard Statements | H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Not dangerouse goods |
| Under the room temperature and away from light | |
| HS Code | 294200 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD 750/kg |
| Use Pattern |
| Triphenylbismuth CAS#: 603-33-8 is added to the fuel of rocket as cruing agent. |
Related Chemicals
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |