TRIS(DIMETHYLAMINO)SILANE CAS#: 15112-89-7; ChemWhat Code: 23957
Identification
| Product Name | TRIS(DIMETHYLAMINO)SILANE |
| Molecular Structure | 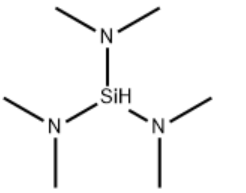 |
| CAS Registry Number | 15112-89-7 |
| EINECS Number | 239-165-0 |
| MDL Number | MFCD00048006 |
| Synonyms | Tris(dimethylamino)silane 15112-89-7 tris(dimethylamino)silicon N,N,N’,N’,N”,N”-Hexamethylsilanetriamine Silanetriamine, N,N,N’,N’,N”,N”-hexamethyl- EINECS 239-165-0 BRN 1901643 MFCD00048006 TDMAS Tris(dimethylamido)silane C6H19N3Si SCHEMBL35636 SCHEMBL2513254 C6-H19-N3-Si DTXSID00884786 BCP30199 [bis(dimethylamino)silyl]dimethylamine s20900 LS-145250 FT-0637184 Silanetriamine, N,N,N,N’,N’,N”,N”-hexamethyl- Silanetriamine, N,N,N’,N’,N”,N”-hexamethyl-;N,N,N’,N’,N”,N”-Hexamethylsilanetriamine |
| Molecular Formula | C6H18N3Si |
| Molecular Weight | 160.31 |
| InChI | InChI=1S/C6H18N3Si/c1-7(2)10(8(3)4)9(5)6/h1-6H3 |
| InChI Key | GIRKRMUMWJFNRI-UHFFFAOYSA-N |
| Isomeric SMILES | CN(C)[Si](N(C)C)N(C)C |
Identification
| Patent Information | ||
| Patent ID | Title | Publication Date |
| KR2021/41804 | Method for manufacturing of alkylaminosilane compound | 2021 |
| KR2017/5305 | PROCESS FOR THE PREPARATION OF TRISALKYLAMINOSILANE | 2017 |
| US9701695 | Synthesis methods for amino(halo)silanes | 2017 |
Physical Data
| Appearance | Colorless liquid |
| Purity | 99.9+%(99.9999%-si) |
| Metal ion | <10ppb |
| Chlorine ion | <1ppm |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 75 – 80 | 5 – 10 |
| 141 | |
| 62 | 45 |
| 142 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 1.058 | ||
| 0.833 | 4 | 24 |
| 0.85 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Spectrum | 1H | ||
| Spectrum | 13C | ||
| Chemical shifts | 13C | tetrahydrofuran-d8 | |
| Chemical shifts | 29Si | CDCl3 | 29.9 |
| Description (IR Spectroscopy) |
| Bands |
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Tris(dimethylamino)silane CAS 15112-89-7
| Conditions | Yield |
| With trichlorosilane; magnesium In ethylene glycol dimethyl ether; n-heptane at 85℃; for 10.5h; Solvent; Temperature; Experimental Procedure Into a 3 L four-neck flask, 87.5 g (3.6 mol) of magnesium and 750 g of n-heptane were charged. An oil bath was heated to 115° C., while stirring the resulting mixture, into a reflux state for 1 hour, and moisture in a solvent and an apparatus was allowed to react with magnesium to dehydrate the solvent and the apparatus, and then the resulting content was cooled with ice water. Into a 500 mL feed tank, a mixture (468 mL) of 408 g (3.01 mol) of TCS and 150 g of DME was charged, and when an internal temperature was decreased to 5° C., 30 mL of a mixture of TCS and DME was charged into the 3 L four-neck flask. In a state in which the temperature was maintained at 10° C. or lower, DMA was fed from a gas phase part of the flask thereinto at a rate of 240 mL per minute for 1 hour.First Reaction Step The oil bath was set to 90° C., and heated until a temperature of a reaction liquid reached 85° C. On the way of the heating, a reaction between DMA hydrochloride and magnesium occurred, but no noticeable rapid increase in temperature was observed. When an internal temperature was maintained at 85° C., TCS was added dropwise into the liquid at a rate of 55 mL per hour, and simultaneously feed of DMA to a gas phase was started at a rate of 420 mL per minute. A time of simultaneous feed of DMA was 8 hours, and a GC analysis of the reaction liquid at completion of the simultaneous feed resulted in 9.3% of DME, 63.0% of n-heptane and a 1D form, 14.8% of a 2D form and 12.9% of TDMAS.Second Reaction Step Further, DMA was fed for 2 hours and 30 minutes. Then, 555 g (12.3 mol) of DMA in total was fed. When reduction of the temperature of the reaction liquid and loss of the 2D form of an intermediate product by the GC analysis were confirmed, feed of DMA was stopped. After cooling, 1950 g of the reaction liquid was obtained.Posttreatment Step The reaction liquid was filtered by a pressure filter, and a residue was washed with 300 g of n-heptane to obtain 1434 g of a filtrate containing TDMAS. When the GC analysis was conducted, 464 g (2.87 mol) of TDMAS was contained therein and a reaction yield was 95%. | 95% |
| With trichlorosilane In cyclohexane |
Safety and Hazards
| Pictogram(s) | |
| Signal | Danger |
| GHS Hazard Statements | H225 (59.38%): Highly Flammable liquid and vapor [Danger Flammable liquids] H226 (40.62%): Flammable liquid and vapor [Warning Flammable liquids] H260 (35.94%): In contact with water releases flammable gases which may ignite spontaneously [Danger Substances and mixtures which in contact with water, emit flammable gases] H261 (64.06%): In contact with water releases flammable gas [Danger Substances and mixtures which in contact with water, emit flammable gases] H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H311 (93.75%): Toxic in contact with skin [Danger Acute toxicity, dermal] H314 (100%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation] H330 (59.38%): Fatal if inhaled [Danger Acute toxicity, inhalation] H331 (34.38%): Toxic if inhaled [Danger Acute toxicity, inhalation] |
| Precautionary Statement Codes | P210, P223, P231+P232, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P284, P301+P317, P301+P330+P331, P302+P335+P334, P302+P352, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P320, P321, P330, P361+P364, P363, P370+P378, P402+P404, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| HS Code | |
| Storage | Under the room temperature and away from light |
| Shelf Life | 1 year |
| Market Price |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 161.322 |
| logP | 0.285 |
| HBA | 3 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 9.72 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Bioactivity |
| In vitro: Efficacy |
| Quantitative Results |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
| Use Pattern |
| TRIS(DIMETHYLAMINO)SILANE CAS#: 15112-89-7 Tris(dimethylamino)silane be used as a CVD precursor material for the deposition of thin film materials such as silicon oxide, silicon nitride, and more. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |