WS 12 CAS#: 68489-09-8; ChemWhat Code: 970687
Identification
| Product Name | WS 12 |
| IUPAC Name | (1R,2S,5R)-N-(4-methoxyphenyl)-5-methyl-2-propan-2-ylcyclohexane-1-carboxamide |
| Molecular Structure | 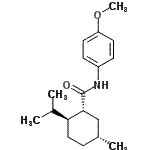 |
| CAS Registry Number | 68489-09-8 |
| EINECS Number | 259-404-2 |
| MDL Number | MFCD11100191 |
| Beilstein Registry Number | No data available |
| Synonyms | CPS-112, (1R,2S,5R)-N-(4-methoxyphenyl)-5-methyl-2-(1-methylethyl)cyclohexanecarboxamide, N-(4-methoxyphenyl)-p-menthane-3-carboxamide, WS-12, WS12, (1R,2S,5R)-N-(4′-methoxyphenyl)-2-isopropyl-5-methylcyclohexanecarboxamide, 2-isopropyl-5-methyl-cyclohexanecarboxylic acid (4-methoxy-phenyl)-amide |
| Molecular Formula | C18H27NO2 |
| Molecular Weight | 289.413 |
| InChI | InChI=1S/C18H27NO2/c1-12(2)16-10-5-13(3)11-17(16)18(20)19-14-6-8-15(21-4)9-7-14/h6-9,12-13,16-17H,5,10-11H2,1-4H3,(H,19,20)/t13-,16+,17-/m1/s1 |
| InChI Key | HNSGVPAAXJJOPQ-XOKHGSTOSA-N |
| Canonical SMILES | C[C@@H]1CC[C@H]([C@@H](C1)C(=O)NC2=CC=C(C=C2)OC)C(C)C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| US2014/90255 | Skin Engaging Member Comprising At Least One Thermally Resilient Sensate | 2014 |
| WO2005/2582 | TRP-P8 ACTIVE COMPOUNDS AND THERAPEUTIC TREATMENT METHODS | 2005 |
| US2005/54651 | COMPOSITIONS AND METHODS FOR THE TREATMENT OF DISEASE ASSOCIATED WITH TRP-P8 EXPRESSION | 2005 |
Physical Data
| Appearance | Powder |
| Solubility | DMSO: >20mg/mL |
| Flash Point | No data available |
| Refractive index | No data available |
| Sensitivity | No data available |
Spectra
| No data available |
Route of Synthesis (ROS)
| Conditions | Yield |
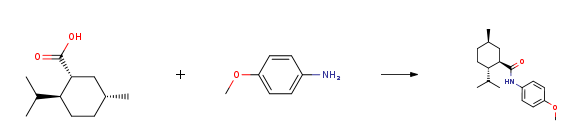
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In ISOPROPYLAMIDE for 14h; Experimental Procedure 1 Example 1; Preparation of Compound IIa4-1 from (-) menthyl chloride. 1.39 grams of Mg metal (57 mmol) was placed in a 100 mL flask and 4 mL of dry THF was added to cover the Mg metal. A crystal of iodine was added to the metal-THF mixture and stirred for several minutes followed by the addition of about 1/3 portion of 10 grams (57 mmol) of (-) menthyl chloride (Aldrich). The mixture was heated to induce Grignard formation and the remaining menthyl chloride (2/3 portion) was added in 50 mL dry THF and stirred until the reaction was complete. The Grignard solution was then canulated under nitrogen into a vessel containing excess dry ice. This dry ice quenched mixture was swirled and poured into 300 mL ice containing 2 mL concentrated HC1. Diethyl ether was added and the mixture separated. The separated organic layers were combined and washed with water then extracted with an aqueous NAOH solution. The aqueous solution (and oil) was acidified with HCl until a solid formed, diethyl ether was added, and the acid was extracted into the organic phase, washed with brine, dried over sodium sulfate, and concentrated to give 4. 18 grams (40%) as white needles. The acid (0.57 grams, 3.1 mmol) was dissolved in 1.5 mL dimethylacetamide (DMA). The PyBOP (2.1 grams, 4.0 mmol), p-anisidine (0.58 grams, 4.7 mmol, Aldrich) and DIPEA (2.7 mL, 15.5 mmol) were added. Another 0.25 grams p-anisidine was added after 2 hours and again after 5 hours. The reaction was diluted with ethyl acetate (EtOAc) after 7 hours, washed twice with 1 N HCl, twice with aqueous sodium bicarbonate, and then with brine. The separated organic layers were combined and dried over sodium sulfate and concentrated to a brown solid. This solid was put through a plug of silica gel with 30% EtOAc/hexanes to remove the color. The product began to crystallize upon concentration, so the crystallization was allowed to occur, the solvent was removed by pipette, and the white needles were washed with cold EtOAc and dried under vacuum to give 0.365 grams (1.26 mmol, 40% yield) of a first crop. Recrystallization of the mother liquor yielded an additional 0.346 grams (1.2 mmol, 39% yield) as white needles. LCMS showed the product to have a molecular weight of 289, corresponding to the desired molecular weight, and the NMR spectra showed it to have the desired structure. | 79% |
Safety and Hazards
| Pictogram(s) |  |
| Signal | Warning |
| GHS Hazard Statements | H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (100%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. |
| Precautionary Statement Codes | P273, P391, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Class 9; Packaging Group: III; UN Number: 3077 |
| Under the room temperature and away from light | |
| HS Code | 290621 |
| Storage | Under the room temperature and away from light |
| Shelf Life | 2 years |
| Market Price | USD |
| Use Pattern |
| WS 12 CAS#: 68489-09-8 has a mint-like cooling taste and can be used as a cooling enhancer in oral care, as a cooling agent in chewing gum and children’s candy. |
| WS 12 CAS#: 68489-09-8 can also be used as a cooling agent for skin and lipstick. |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Warshel Chemical Ltd | http://www.warshel.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |