1,5-HEXADIENE CAS#: 592-42-7; ChemWhat Code: 32392
Identification
| Product Name | 1,5-HEXADIENE |
| IUPAC Name | hexa-1,5-diene |
| Molecular Structure | |
| CAS Registry Number | 592-42-7 |
| EINECS Number | 209-754-7 |
| MDL Number | MFCD00008666 |
| Synonyms | 1,5-HEXADIENE 592-42-7 Hexa-1,5-diene Diallyl Biallyl Hexadiene (DOT) alpha,omega-Hexadiene .alpha.,.omega.-Hexadiene EINECS 209-754-7 UNII-4MTZ4764FI NSC 60690 4MTZ4764FI DTXSID4049323 NSC-60690 CH2=CHCH2CH2CH=CH2 DTXCID9029279 alpha,omegaHexadiene inchi=1/c6h10/c1-3-5-6-4-2/h3-4h,1-2,5-6h pygskmbevaiccr-uhfffaoysa-n un2458 1,5-Hexadiene, 97% CHEMBL31747 BCP08456 NSC60690 Tox21_202852 MFCD00008666 AKOS015960557 NCGC00260398-01 CAS-592-42-7 H0084 NS00020101 EN300-105621 F14840 A832222 Q161551 25067-96-3 |
| Molecular Formula | C6H10 |
| Molecular Weight | 82.14 |
| InChI | InChI=1S/C6H10/c1-3-5-6-4-2/h3-4H,1-2,5-6H2 |
| InChI Key | PYGSKMBEVAICCR-UHFFFAOYSA-N |
| SMILES | C=CCCC=C |
| Patent Information | ||
| Patent ID | Title | Publication Date |
| CN110981836 | Method for preparing diepoxide by one-pot method | 2020 |
| US2018/282866 | RUTHENIUM PRECURSOR, PREPARATION METHOD THEREFOR AND METHOD FOR FORMING THIN FILM USING SAME | 2018 |
| CN103588807 | A double ( alkane oxygen silicon-based ) alkane preparation method | 2016 |
| WJP5652360 | Method of manufacturing Organoxysilane compd. | 2015 |
| US2013/35451 | Solid State Polymerization Process for Polyester with Phosphinic Acid Compounds | 2013 |
| US8772533 | Organoleptic compound | 2014 |
| US6870068 | Synthesis of pentafluorosulfuranyl substituted alkanes | 2005 |
| US2004/82803 | Trichlorosilyl groups containing organochlorosilanes and their preparation methods by the double-silylation of olefins with trichlorosilane | 2004 |
Physical Data
| Appearance | Colorless to light yellow transparent liquid |
| Melting Point, °C |
| -139.5 |
| -141.2 |
| -140.8 |
| -140.68 |
| Boiling Point, °C | Pressure (Boiling Point), Torr |
| 59.99 | 750.075 |
| 59 – 60 | |
| 58.5 – 59.5 | |
| 59.7 | |
| 60 – 62 | |
| 59.5 | 760 |
| Density, g·cm-3 | Reference Temperature, °C | Measurement Temperature, °C |
| 0.6886 | 25 | |
| 0.6909 | 4 | 20 |
| 0.6925 | 4 | 25 |
| 0.6911 | 4 | 20 |
| 0.6914 | 4 | 20 |
| 0.7031 | 4 | 20 |
Spectra
| Description (NMR Spectroscopy) | Nucleus (NMR Spectroscopy) | Solvents (NMR Spectroscopy) | Temperature (NMR Spectroscopy), °C |
| Chemical shifts, Spectrum | 1H | benzene-d6 | 25 |
| Chemical shifts | 1H | [D3]acetonitrile | |
| Chemical shifts | 13C | [D3]acetonitrile | |
| Spectrum | 1H | ||
| Chemical shifts | 1H | tetrahydrofuran-d8 | |
| Chemical shifts | 13C | tetrahydrofuran-d8 | |
| Chemical shifts | 1H | [(2)H6]acetone |
| Description (IR Spectroscopy) | Solvent (IR Spectroscopy) | Temperature (IR Spectroscopy), °C |
| Spectrum | ||
| Spectrum | gas | |
| Spectrum | neat (no solvent) | |
| Spectrum | neat (no solvent) | |
| Bands | solid matrix | -163.2 |
| Spectrum | gaseous matrix | -261.2 |
| Description (UV/VIS Spectroscopy) | Solvent (UV/VIS Spectroscopy) | Comment (UV/VIS Spectroscopy) |
| Spectrum | 165 – 195 nm, Dampf. | |
| Spectrum | Ratio of solv200 – 215 nm | |
| Spectrum | cyclohexane |
Route of Synthesis (ROS)
| Conditions | Yield |
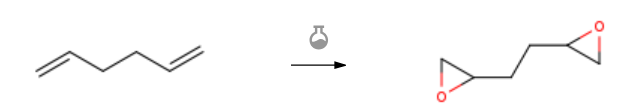
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 16h; Experimental Procedure (±)-((2S,5S)-tetrahydrofuran-2,5-diyl) dimethanol 6a,((2R,5R)-tetrahydrofuran-2,5-diyl) dimethanol 6b: and((2R,5S)-tetrahydrofuran-2,5-diyl) dimethanol 6c from 1,5-hexadiene 17 through the 1,5-diepoxide 18: 3-Chloroperoxybenzoicacid (57-60%, 48.6 g, 281 mmol) was addedin five portions to a mechanically-stirred solution of 1,5-hexadiene 17 (4.84 g, 59 mmol) in CH2Cl2 (150 mL) whilecooling (0°C). The reaction mixture was allowed to warm toroom temperature then stirring was continued (16h). Saturatedaqueous sodium bicarbonate (100 mL) was then addedto the reaction mixture while stirring followed by separationof the organic layer and washing with 1M aqueous KOH (7 x50 mL). The organic layer was then dried over anhydrousMgSO4, filtered and concentrated to provide the racemic/meso 1, 5-diepoxides 18 (6.8 g, >99%). A mixture ofaqueous NaOH (18 mL, 0.1M) and the diepoxide 18 (1.0 g,8.7 mmol) was stirred at room temperature (16 h). Water wasthen removed and the oily residue was flushed through asilica gel column giving the mixture of oily (2S,5S)-6a,(2R,5R)-6b and (2S,5R)-6c diols (0.8 g, 70%). The mixturewas then submitted to gravity-column chromatography(CHCl3/tert-butanol, 7/3) which separated the more mobile(2S,5R)-6c from the racemic mixture of 6a/6b. Acetylation(Ac2O/pyridine) of a portion of the 6a/6b/6c mixture followedby flash chromatography gave a mixture of the threecorresponding stereoisomeric diacetates. The diacetates weresubmitted to chiral HPLC (ChiracelOJ, 4.6 x 250 mm, 10;mobile phase 80% hexane/20% ethanol; 0.7 mL/min, 22°C)and indicated that the ratio of racemic: meso diacetates was54:46. | 99% |
| With 3-chloro-benzenecarboperoxoic acid at 10 – 20℃; for 19h; Experimental Procedure 1 A one-pot method for preparing diepoxide includes the following steps: S1. Epoxidation reaction: At a low temperature, add 10.04g1,5-hexadiene to the reactor, slowly add m-chloroperoxybenzoic acid solution dropwise, control the drop acceleration to make the system temperature around 10 , and maintain after the dropwise addition The reaction was carried out at a low temperature for 4h, and then the temperature was raised to 20°C, the reaction was continued for 15h, and the reaction was completed to obtain a crude diepoxyhexane solution, in which the molar ratio of diene to m-chlorobenzoyl chloride was 1:2.45;S2. Separation and purification: add 125g of 20% mass fraction sodium sulfite aqueous solution to the crude diepoxyhexane solution, keep the system temperature at 20°C, reduce the reaction for 2h, extract and separate the organic phase. To the organic phase, add 165g of a 20% sodium bicarbonate mass fraction, maintain the system at 20°C, neutralize the reaction for 3 hours, and extract the organic phase. The organic phase was washed three times with water (150 mL×3), the organic phase was extracted and separated, dried over anhydrous sodium sulfate, the solid filter residue was filtered off, the filtrate was distilled under reduced pressure, the solvent methylene chloride was recovered, and the product was collected to obtain diepoxyhexane.Among them, the preparation method of m-chloroperoxybenzoic acid solution is as follows:Add 50.4g of potassium hydroxide, 260mL of water, 100g of 30% hydrogen peroxide solution, 1.5g of magnesium sulfate heptahydrate to the reactor, stir to dissolve, then add 300mL of dioxane, control the feeding temperature at 15 , in the fierce Under stirring, 52.5 g of m-chlorobenzoyl chloride was added all at once, maintaining the reaction temperature at 20° C., and stirring for 20 min. Cool down, add 20% sulfuric acid solution, control the temperature of the system at 10 , acidify to pH 2, add 400mL of dichloromethane, extract and separate the organic phase, dry with anhydrous potassium sulfate, filter off the solid filter residue to obtain m-chloroperoxy Benzoic acid / dichloromethane solution,The molar ratio of chlorobenzoyl chloride, potassium hydroxide and hydrogen peroxide is 1: 3: 2.93.The yield of diepoxyhexane is 95.1% and the purity is 93.4%. | 95.1% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane | 87% |
Safety and Hazards
| Pictogram(s) |    |
| Signal | Danger |
| GHS Hazard Statements | H225 (100%): Highly Flammable liquid and vapor [Danger Flammable liquids] H304 (90.4%): May be fatal if swallowed and enters airways [Danger Aspiration hazard] H315 (28.1%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (30%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (37.8%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] |
| Precautionary Statement Codes | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P280, P301+P316, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P331, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
Other Data
| Transportation | Under the room temperature and away from light |
| Storage | Under the room temperature and away from light |
| Shelf Life |
| Druglikeness | |
| Lipinski rules component | |
| Molecular Weight | 82.1454 |
| logP | 3.022 |
| HBA | 0 |
| HBD | 0 |
| Matching Lipinski Rules | 4 |
| Veber rules component | |
| Polar Surface Area (PSA) | 0 |
| Rotatable Bond (RotB) | 3 |
| Matching Veber Rules | 2 |
| Toxicity/Safety Pharmacology |
| Quantitative Results |
Buy Reagent | |
| No reagent supplier? | Send quick inquiry to ChemWhat |
| Want to be listed here as a reagent supplier? (Paid service) | Click here to contact ChemWhat |
Approved Manufacturers | |
| Watsonnoke Scientific Limited | http://www.watsonnoke.com/ |
| Want to be listed as an approved manufacturer (Requires approvement)? | Please download and fill out this form and send back to approved-manufacturers@chemwhat.com |
Other Suppliers | |
| Watson International Limited | Visit Watson Official Website |
Contact Us for Other Help | |
| Contact us for other information or services | Click here to contact ChemWhat |